The European Union (EU) has announced they will be implementing new restrictions on retinol in skincare products. Strategy Skin’s guiding principles for achieving healthy skin have always been grounded in safety and provident scientific research. As creators of the next-generation retinoid formula, The A Retinal Serum, we’re excited to share the details of the EU's regulatory changes with you.
In the realm of skincare, innovation and effectiveness go hand in hand, and The A Retinal Serum stands as a testament to this synergy. Crafted by our visionary co-founders Amanda Hlatky and Mara Jenkins, this multi-corrective serum is a culmination of three decades of expertise in advanced skin therapy and global education. The result? A groundbreaking product that redefines the approach to aging and skin health, all while preemptively adhering to the latest EU regulations on vitamin A in cosmetic skincare products.

NAVIGATING EU REGULATIONS: A COMMITMENT TO SAFETY AND EFFICACY
The European Union, known for its stringent safety standards, has recently set new restrictions on the use of vitamin A, specifically its Retinol Equivalent (RE), in cosmetic products. The new restrictions also include the cosmetic grade vitamin A derivatives, Retinyl Acetate and Retinyl Palmitate — also known as "Retinyl Esters."
These regulations are a response to concerns about the overall exposure of consumers to vitamin A, taking into account contributions from food and food supplements. The Scientific Committee on Consumer Safety (SCCS) has deemed concentrations of 0.05% RE in body lotions, and 0.3% RE for face and other leave-on and rinse-off products as safe. These guidelines underscore the importance of safe concentrations of vitamin A in skincare, ensuring consumer safety and well-being.
THE SCIENCE BEHIND THE MAGIC: OUR NEXT-GENERATION RETINOID
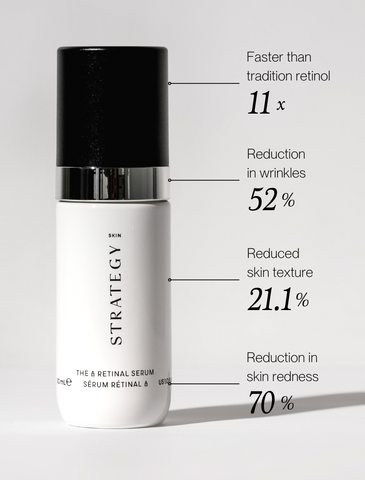
At the heart of The A Retinal Serum lies retinaldehyde (retinal), a stable and potent form of vitamin A, expertly delivered deep into the skin's living layers. This isn’t just any retinal; it’s sophisticatedly encapsulated, ensuring each molecule is stabilized until it safely interacts with the epidermis. What sets retinal apart is its rapid signaling for cellular renewal, outpacing traditional retinol by 11 times, with the added benefit of regulating excess molecules within the skin itself. This ingenious mechanism equates to less irritation and a harmonious balance with the skin’s delicate moisture barrier and microbiome.
STANDING TALL: COMPLIANCE AND EXCELLENCE
The A Retinal Serum not only adheres to the new EU regulations but also stands as an excellent alternative to other retinoids. Its unique formulation ensures that it delivers the benefits of vitamin A without contributing significantly to the overall exposure concerns highlighted by the SCCS. This makes The A Retinal Serum a safer choice, especially for those aiming to navigate the complex world of skincare ingredients with confidence and assurance.

UNVEILING THE RESULTS: CLINICALLY PROVEN EFFICACY
The A Retinal Serum isn’t just a product; it’s a promise of transformation. Clinically proven to deliver, our encapsulated retinaldehyde, it boasts a 21.1% improvement in skin texture, a remarkable 52% reduction in wrinkles, and a 70% decrease in erythema (redness).
Yes — you read that right — it actually reduces skin irritation and redness. A feat for any skincare product, but especially a retinoid-forward formulation. As any retinol user will know, reducing skin redness and sensitivity is not typically associated with the use of retinoids. Typically, retinoid use can result in side effects that are quite the opposite, including irritation and dryness known as retinization. These impressive results are a testament to The A Retinal Serum's unique formulation and the power of retinal, all while ensuring compliance with the highest safety standards.
 *Based on a 4-week, double blind clinical trial
*Based on a 4-week, double blind clinical trial
KEY FINDINGS FROM THE EU'S NEW RESTRICTIONS ON RETINOL
It’s important to note that retinol and its fellow vitamin A derivatives, retinyl palmitate and retinyl acetate, are not being banned in the EU. They are still deemed safe, and vitamin A-derived retinoids are still determined to be the most effective anti-aging topical solution for skin rejuvenation.
According to the SCCS, these retinoids are being restricted to further assist the European Food Safety Authority (ESFA) in protecting the approximate 5% of EU citizens already at risk of, or who are currently exceeding, the upper limits of vitamin A intake from diet and food supplements compared to what is recommended by the ESFA. The SCCS also acknowledges in their revision that “compared to food, the contribution of vitamin A from cosmetics is lower”, however, the SCCS can only consult on cosmetic regulation in support of the EFSA.
Here are the key takeaways:
- Retinol and Retinyl Esters will be restricted to a maximum concentration in face creams and hand creams of 0.3% and a maximum concentration in body lotions of 0.05%
- These changes are aimed at minimizing the risks associated with overexposure to retinoids in cosmetic products
- According to findings by the SCCS, retinol and retinyl esters, have a higher potential for absorption into the bloodstream compared to retinaldehyde, which primarily exerts its effects locally in the skin
EMBRACING THE FUTURE OF SKINCARE
The A Retinal Serum stands as a beacon of innovation, offering a strategy for youth and vitality in every drop. It’s a product born from experience, expertise, and a relentless pursuit of perfection, aligning with the latest EU regulations and setting a new standard in skincare. You can embrace The A Retinal Serum and transform your skincare journey, knowing you are choosing a product that is not only effective but also safe and compliant.
SCCS Reference(s):
SCCS/1639/21 Final version Scientific Committee on Consumer Safety SCCS REVISION of the scientific Opinion (SCCS/1576/16) on vitamin A (Retinol, Retinyl Acetate, Retinyl Palmitate). 2023. Scientific Committee on Consumer Safety (SCCS). https://health.ec.europa.eu/system/files/2023-08/sccs_o_261.pdf via https://health.ec.europa.eu/scientific-committees/scientific-committee-consumer-safety-sccs_en

Leave a comment